|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Battery Smarts
Batteries 101. All batteries operate by turning chemical reactions into electricity. They're like little energy conversion factories. While you don't need to know all the technical details to make better battery choices, a little understanding goes a long way. Everything important is in this short article. After you read it, you'll be able to "talk tech" with the best of them. You can even make your own battery with an ordinary potato and two thin strips of metal (one zinc and one copper). They represent the three main things you need to make any kind of battery: (1) an anode, (2) a cathode, and (3) an electrolyte. WAIT! Don't go away! This will get interesting soon… I promise. I know this kind of anode cathode electrolyte talk is giving you bad flashbacks to high school chemistry class. However, unlike Mister Beaker's chem class, this one has no mid-term exams, and you might learn something you can apply in real life. However… if you're already getting cold sweats and palpitations, just skip to the next section.
The potato battery really works, but it's far from state-of-the-art. You can power a small digital clock or other very low-drain device with it, but not much else. Besides, you'd look very silly with a potato strapped to your cell phone. Fortunately, there are better ways to build a battery. By choosing anode and cathode materials with high "electromotive force," stronger electrolytes, and better packaging, manufacturers have packed a lot of power in a small, lightweight package. There's been a ton of trial and error along the way – ever since Count Alessandro Volta invented the first battery over two hundred years ago. Tens of thousands of combinations have been tried, some with disastrous results (mainly explosions). Most people feel we're now close to having developed the ultimate in battery technology – considering all factors: cost, reliability, long life, safety, energy density, etc. The ten different technologies described in the rest of this article pretty much represent the full range (although each entails its own set of tradeoffs). Future breakthroughs will probably come from mini fuel cells or other kinds of energy conversion devices. But anything is possible… The Taxonomy of Batteries 1. Zinc-Carbon. 2. Alkaline. You don't have to use acid for a battery electrolyte. Sorry to keep making you think back to chem class, but what's the opposite of an acid? That's right… alkaline. Well, using an alkaline electrolyte (instead of acid) makes all the difference. An alkaline battery uses the same anode and cathode materials as a plain zinc-carbon battery – but the alkaline electrolyte gives it five times the life and a flatter (better) discharge curve. Just as important, alkalines are much less likely to leak. Not perfect though. If the alkaline zinc-chloride electrolyte does leak out, it can still ruin your equipment. [Here's a tip: use ordinary white vinegar on a Q-Tip to neutralize the corrosion in your electronics. I've brought many a tape recorder, answering machine, and flashlight back to life this way].
3. Zinc-Air. 4. Nickel Cadmium. NiCad's were the first, and remain the most common kind of rechargeable battery. They use a nickel cathode and a cadmium anode. That's a problem. You may not know this, but cadmium is a highly toxic metal. Ingest some and you're in big trouble. I'm not all that worried you're going to swallow an AA battery (although kids have been known to try), but there's concern the NiCad's thrown in the trash will end up in an incinerator or landfill. If your town has a battery recycling program (where I live it's called STOP: Stop Throwing Out Pollutants), please use it to recycle your NiCad and other batteries. Whole Foods Market, Ikea, and other some environmentally-sensitive retailers have collection barrels in their stores. Manufacturers such as Black & Decker have recycling programs for the specialized NiCad batteries used in appliances like DustBusters. You can also contact the Rechargeable Battery Recycling Corporation (RBRC) at 800-822-8837 for more info. The main reason why I don't like NiCads is their susceptibility to the dreaded "memory effect." I'm sure you've seen this happen: your electric shaver, DustBuster, camcorder, or cordless phone seems unable to hold a charge anymore. You'll take it off the charger and it'll run OK for a short while and then abruptly quit. That's the memory effect at work. If a NiCad is only partially discharged, then put back on its charger a few times in a row, it will "forget" how to hold a full charge. (For you chem class whiz kids who want to know why this happens: potassium-hydroxide crystals form inside the battery cells, blocking the chemical reactions and the flow of electricity.) There are two ways to prevent memory effect. One is to always let your device run all the way down before recharging it (a pain in the neck). The second is to get a "smart" recharger (like the Accumanager). A smart recharger or "conditioner" will automatically suck all the juice out of a NiCad before charging it back up again. Besides memory effect and toxic metal pollution, there's another reason to dislike NiCads: a crappy discharge curve. Even a new NiCad, fully charged, will only reliably give you back about two-thirds of its power. After that point, voltage drops sharply. Your digital camera, camcorder, or PDA will either quit suddenly, or perform erratically. Either behavior is unacceptable. Luckily, better alternatives are now on the market. 5. Nickel Metal Hydride. One of the first "advanced technology" rechargeables, Nickel Metal Hydride batteries (abbreviated Ni-MH, and pronounced "nymph" like the sea creature) are getting a lot of traction these days. They are superior to Ni-Cads in every way except two. They hold a better charge, last up to 40% longer, don't contain toxic metal, and don't suffer memory effect nearly as much. There are only two minuses: Ni-MH's cost a little more than NiCads and can only be recharged about 600 times compared to 800 or more for NiCads. For the chemists: NiMH's use a hydrogen-storing alloy metal as their anodes, a nickel oxide cathode. First available only built-in to higher-end electronics like PDA's and laptop computers, NiMH's are now available in common sizes like AA, AAA, C, and D cells. These can be recharged in the same "wall wart" recharger you've been using for NiCads, although a smart charger/conditioner like the Accumanager or AlphaPower BC-900 is better still. A conditioner has the smarts to monitor voltage while it's charging, and adjust the flow of electricity to prevent overheating/overcharging (which will dramatically shorten the life of your NiMH batteries). A good charger will also switch to "trickle" mode after bringing your cells to full charge. This tiny trickle of electricity will top off the batteries and keep them fully charged until you remove them from the charger. That's important for rechargeables, because all types have a much higher self-discharge rate than one-use batteries. This self-discharge effect is the main drawback of NiMH cells, and the main reason I don't put regular ones in flashlights or other infrequently used devices. Just a month on the shelf, and the unused batteries have already lost much of their charge. Nonetheless, NiMH's are great in digital cameras, PDA's, and other things you use all the time. I've even done surgery on my electric shaver, performing a "Nicadectomy" and transplanting a NiMH double-A cell into its guts. 6. Rechargeable Alkaline Manganese. Built with similar chemistry to regular alkaline batteries, these can be recharged about a dozen to two dozen times, although their capacity will diminish with each recharge. Nonetheless, they're sometimes a better rechargeable choice than Ni-Cads or NiMH's. That's because alkalines have a much longer shelf life and higher voltage (1.5v). They're a natural in flashlights, toys, radios, and other lightly-used items. You'll need a special, high-end recharger though, like the AccuManager. Whether you choose NiMH's or rechargeable alkalines, you are going to save a lot of money during the life of the batteries. They're more expensive to buy, but much, much cheaper in the long run. Throwaway alkalines cost about ten cents an hour (each) to use, but rechargeables can be renewed for only a penny's worth of electricity.
8. Gelled Lead-Acid. 9. Lithium-Ion. I'm typing this article on a laptop computer powered by a Li-Ion (pronounced "Lion") battery. Lithium is a natural for power-hungry portable electronics. It's the lightest solid substance on earth. It's also the metal with the strongest positive emf (electromotive force) and therefore makes batteries with the highest known energy density (greatest amount of power per pound of weight). In a Li-Ion battery, the anode is plain carbon, the cathode is cobalt oxide, and the electrolyte is composed of lithium salts in ether. Manufacturers don't use pure lithium for a really good reason. It explodes. Pour plain water on lithium powder and KABOOM! You better go find a "Class D" fire extinguisher (for reactive metals) before things really get bad. A regular extinguisher like you probably have on the wall (Class "A" "B" or "C") is water-based and will just make the fire worse. Even manufacturing pure lithium battery cells is difficult and dangerous. If molten lithium comes in contact to the cobalt oxide cathode, you better stand back. Far back. Really far back. Understandably, most manufacturers play it safe and use "extracted lithium ions" instead of pure lithium. Somewhat less power but far more safety. If you hold a lithium cell in your hand, you'll be amazed at how light it is. The battery actually feels like a hollow shell, even though it packs more power than a heavy regular cell. Li-Ion batteries are commonly available in AA size and in 9-volt "transistor radio" size. The latter are sold as "super long-life" batteries for smoke detectors. But Li-Ion cells are available in many specialty sizes, like button batteries, and in machine-specific configurations for digital cameras and the like. They come in one-use or rechargeable varieties. As a rechargeable, modern Li-Ion's don't suffer from memory effect and are pretty forgiving of poor recharging habits. You'll find them in many high-end portable electronic devices sold these days: MP3 players, camcorders, PDA's, etc. 10. Lithium Polymer. Battery Technology Summary:
|



 Back to our potato battery. If you poke the zinc strip into one end of the
potato, it'll become an anode. Anode just means "negative terminal." Of course,
you know that batteries have + (positive) and - (negative) ends. Anodes chuck
off electrons and start them flowing on down the line (to power your electronic
device). Stick the copper strip in, and it becomes the cathode. Cathode just
means "positive terminal." It takes in the electrons as they return from their
journey through your electronic device. The potato is mildly acidic and
functions as an electrolyte. Like the acid in your car battery, the potato
completes the electric circuit and promotes the transfer of electrons.
Back to our potato battery. If you poke the zinc strip into one end of the
potato, it'll become an anode. Anode just means "negative terminal." Of course,
you know that batteries have + (positive) and - (negative) ends. Anodes chuck
off electrons and start them flowing on down the line (to power your electronic
device). Stick the copper strip in, and it becomes the cathode. Cathode just
means "positive terminal." It takes in the electrons as they return from their
journey through your electronic device. The potato is mildly acidic and
functions as an electrolyte. Like the acid in your car battery, the potato
completes the electric circuit and promotes the transfer of electrons. 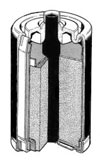 These are the "corner store" batteries of my youth. They were all
we had back then and represent the bottom of the line today. Zinc-Carbons (ZC's)
were the first "dry cells." A wet cell is like your car battery – full of liquid
sulfuric acid sloshing around – and therefore not a good choice for your
flashlight or PDA. Way back when, people were really excited when dry cells
became the first available "household battery." They use zinc anodes and
manganese dioxide as the
cathode. {Stop freaking out about the chemical names you lightweight!} The electrolyte is sulfuric acid, although it's in a non-liquid state; powdered or gelled. The anode and cathode are formed as concentric
cylinders, with an insulating sleeve between them. In a perfect world, the zinc
anode will be "digested" evenly from the inside out as the battery is
discharged. However, what often happens is the process doesn't happen evenly as
it should, and the acid eats right through the zinc casing and insulator,
leaking out and (usually) ruining the device it's in. This is a major drag. It's
bad enough to lose a flashlight or radio, but with portable electronic gear
costing hundreds of dollars, trying to save a couple of bucks buying cheap
zinc-carbon batteries seems downright foolish. There's another reason why ZC's
are on the endangered list: they don't have much staying power, and when they
begin to run down, their power curve is ugly. Most single cells have the same
output voltage when new: either 1.5 volts for "primary batteries" (ones that
can't be recharged) or 1.25 volts for "secondaries" (rechargeables). Most
batteries will maintain that voltage until the bitter end. Not ZC's. As they
discharge, the voltage falls lower and lower. If you're powering a flashlight,
this is just annoying as the beam becomes dimmer and dimmer. However, modern
electronics are less forgiving about their power, and hate voltage fall-off. In
the "best" case, they'll shut themselves off. Worst case, they'll start acting
erratically or lock up. For all these reasons, ZC's are becoming hard to find in
stores. Usually you'll encounter them supplied by producers of TV remote
controls, cheap toys, etc. My advice. Throw them away (better: recycle them if
your town does a hazardous materials collection) and put in some good alkalines.
These are the "corner store" batteries of my youth. They were all
we had back then and represent the bottom of the line today. Zinc-Carbons (ZC's)
were the first "dry cells." A wet cell is like your car battery – full of liquid
sulfuric acid sloshing around – and therefore not a good choice for your
flashlight or PDA. Way back when, people were really excited when dry cells
became the first available "household battery." They use zinc anodes and
manganese dioxide as the
cathode. {Stop freaking out about the chemical names you lightweight!} The electrolyte is sulfuric acid, although it's in a non-liquid state; powdered or gelled. The anode and cathode are formed as concentric
cylinders, with an insulating sleeve between them. In a perfect world, the zinc
anode will be "digested" evenly from the inside out as the battery is
discharged. However, what often happens is the process doesn't happen evenly as
it should, and the acid eats right through the zinc casing and insulator,
leaking out and (usually) ruining the device it's in. This is a major drag. It's
bad enough to lose a flashlight or radio, but with portable electronic gear
costing hundreds of dollars, trying to save a couple of bucks buying cheap
zinc-carbon batteries seems downright foolish. There's another reason why ZC's
are on the endangered list: they don't have much staying power, and when they
begin to run down, their power curve is ugly. Most single cells have the same
output voltage when new: either 1.5 volts for "primary batteries" (ones that
can't be recharged) or 1.25 volts for "secondaries" (rechargeables). Most
batteries will maintain that voltage until the bitter end. Not ZC's. As they
discharge, the voltage falls lower and lower. If you're powering a flashlight,
this is just annoying as the beam becomes dimmer and dimmer. However, modern
electronics are less forgiving about their power, and hate voltage fall-off. In
the "best" case, they'll shut themselves off. Worst case, they'll start acting
erratically or lock up. For all these reasons, ZC's are becoming hard to find in
stores. Usually you'll encounter them supplied by producers of TV remote
controls, cheap toys, etc. My advice. Throw them away (better: recycle them if
your town does a hazardous materials collection) and put in some good alkalines. These were the first button batteries, and are still popular for
hearing aids. They've found new application as an emergency single-use power
supply for your cell phone. They come in a sealed foil pouch. When you open the
pouch, air activates the battery and it will keep your cell alive until you get
back to civilization.
These were the first button batteries, and are still popular for
hearing aids. They've found new application as an emergency single-use power
supply for your cell phone. They come in a sealed foil pouch. When you open the
pouch, air activates the battery and it will keep your cell alive until you get
back to civilization.  7. LSD Battery. These are really far out man. Invented by Timothy Leary, these... wait. Strike that. Invented by Sanyo Electric Co. in 2005, these are next-generation nickel metal hydride batteries. Rather than lysergic acid diethylamide, LSD actually stands for "Low Self Discharge" and hold their charge far longer than regular Ni-MH cells. After a full year on the shelf, Sanyo's "Eneloop" batteries still retain 85% of their full charge, making them suitable for all sorts of applications you wouldn't have considered before: TV remote controls, clocks, and flashlights. They're also great in digital cameras, not only because they'll retain full charge longer, but also because of LSD's superior electrical performance in high current drain applications such as cameras and electronic flashes. For you techies, advantage is primarily due to a high-performance negative electrode "superlattice alloy." There's more to love about the
7. LSD Battery. These are really far out man. Invented by Timothy Leary, these... wait. Strike that. Invented by Sanyo Electric Co. in 2005, these are next-generation nickel metal hydride batteries. Rather than lysergic acid diethylamide, LSD actually stands for "Low Self Discharge" and hold their charge far longer than regular Ni-MH cells. After a full year on the shelf, Sanyo's "Eneloop" batteries still retain 85% of their full charge, making them suitable for all sorts of applications you wouldn't have considered before: TV remote controls, clocks, and flashlights. They're also great in digital cameras, not only because they'll retain full charge longer, but also because of LSD's superior electrical performance in high current drain applications such as cameras and electronic flashes. For you techies, advantage is primarily due to a high-performance negative electrode "superlattice alloy." There's more to love about the  Same rechargeable technology as your car battery, but with a sealed or jelled
sulfuric acid electrolyte instead of the open liquid acid sloshing around.
They're old tech but still good for special applications. Most likely, the
backup battery in your home or business alarm system is a lead-acid. So is the
battery in your computer UPS (uninterruptible power supply). Lead-acid cells are
very forgiving. They tolerate partial discharges without losing capacity (no
memory effect), can be kept topped-off with a full charge with a simple (cheap)
charging device, and come in big, powerful sizes. They're not available in
"normal" sizes though; no AA's, C's, D-cells, etc. Please be sure to bring these to a recycler when they're dead and gone.
Same rechargeable technology as your car battery, but with a sealed or jelled
sulfuric acid electrolyte instead of the open liquid acid sloshing around.
They're old tech but still good for special applications. Most likely, the
backup battery in your home or business alarm system is a lead-acid. So is the
battery in your computer UPS (uninterruptible power supply). Lead-acid cells are
very forgiving. They tolerate partial discharges without losing capacity (no
memory effect), can be kept topped-off with a full charge with a simple (cheap)
charging device, and come in big, powerful sizes. They're not available in
"normal" sizes though; no AA's, C's, D-cells, etc. Please be sure to bring these to a recycler when they're dead and gone.  A close cousin of the Li-Ion, the Polymer Lithium-Ion
Battery (PLB) have a unique shape. They often look like a thin foil food
package. But don't put a PLB in the microwave. Heat is their worst enemy. It
causes them to swell up, with ruinous consequences. PLB's are composed of
multiple dry film-like layers in a plastic or laminated aluminum foil package
(instead of the semi-liquid constituents encased in a rigid metal can typical of
every other battery described so far). This non-rigid construction using dry
polymer electrolyte/separator films allows PLB's to be molded into a variety of
custom shapes and built-in to smaller portable electronics (for example, the
iPod).
A close cousin of the Li-Ion, the Polymer Lithium-Ion
Battery (PLB) have a unique shape. They often look like a thin foil food
package. But don't put a PLB in the microwave. Heat is their worst enemy. It
causes them to swell up, with ruinous consequences. PLB's are composed of
multiple dry film-like layers in a plastic or laminated aluminum foil package
(instead of the semi-liquid constituents encased in a rigid metal can typical of
every other battery described so far). This non-rigid construction using dry
polymer electrolyte/separator films allows PLB's to be molded into a variety of
custom shapes and built-in to smaller portable electronics (for example, the
iPod).